Varicella Vaccine Efficacy And Safety
Early studies in healthy children in Japan showed that the vaccine was safe and produced strong, persisting immunity . In the USA, a randomized controlled trial of high-potency varicella vaccine conducted among 914 healthy children with a mean age of 4.7 years showed a vaccine efficacy of 100% at 1 year and 98% at 2 years, or 92% in households with a case of varicella . In most early dose-ranging and efficacy trials, the vaccine gave a high degree of protection against varicella, but some vaccine failures occurred, notably in very young children, children with asthma or eczema and children on corticosteroid treatment . Importantly, clinical trials among children with acute leukemia or other cancers showed that vaccination was safe for patients among whom chemotherapy was suspended who had acceptable lymphocyte counts or were in remission .
Varicella vaccines were first licensed in Germany and Sweden in 1984 , Japan, and Korea in 1988 and the USA in 1995 . The currently licensed monovalent vaccines Varivax and Varilrix were derived from the seed vOka by additional passaging in cell culture . Two combined measles-mumps-rubella-varicella live-attenuated vaccines were also developed to enable more streamlined integration with existing childhood vaccination schedules . ProQuad/Merck was licensed by the US FDA in 2005 for children aged 12 months to 12 years on the basis of safety and non-inferior immunogenicity compared with MMR and monovalent varicella vaccines .
Mild Side Effects Of Shingles Vaccine:
- Redness, soreness, swelling, or itching at the site of the injection .
It is safe to be around infants and young children, pregnant women, or people with weakened immune systems after you get the shingles vaccine. There is no documentation of a person getting chickenpox from someone who has received the shingles vaccine .
Some people who get the shingles vaccine will develop a chickenpox-like rash near the place where they were vaccinated. As a precaution, this rash should be covered until it disappears.
Like all vaccines, shingles vaccine is being closely monitored for unusual or severe problems by CDC and FDA.
Signs of a severe allergic reaction can include hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, and weakness. These would start a few minutes to a few hours after the vaccination. If you have a severe allergic reaction or other emergency that cant wait, call 9-1-1 or get the person to the nearest hospital. Otherwise, call your doctor.
Afterward, the reaction should be reported to the Vaccine Adverse Event Reporting System . Your doctor might file this report, or you can do it yourself through the VAERS website, or by calling 1-800-822-7967.
The shingles vaccine does not contain thimerosal .
This information was taken directly from the Shingles Vaccine Information Statement dated 10/06/2009.
For more information on possible side effects from vaccination, visit CDCs Possible Side Effects from Vaccines page.
What Are The Main Differences Between Shingrix And Zostavax
Shingrix is a recombinant, adjuvanted zoster vaccine that was first FDA-approved in 2017. It uses the varicella-zoster glycoprotein E antigen to produce an immune response in the body. An adjuvant, or added ingredient, helps boost the bodys immune response to the virus. Because Shingrix is an inactivated vaccine, it can be used in immunocompromised patients or those with a weakened immune system.
Shingrix is administered as an injection into the muscle . It is given in two separate doses with a period of two to six months in between. The second dose is necessary to ensure long-term effectiveness.
Zostavax, approved in 2006, is a live attenuated herpes zoster vaccine. In other words, Zostavax contains a weakened version of the actual virus to produce an immune response. For this reason, it is not recommended for those who are immunocompromised. Or else, the vaccine itself could cause an infection.
Zostavax is administered as a single injection underneath the skin . It comes in a frozen version and a refrigerator-stable version. The frozen version must be kept frozen during transport and storage to ensure its effectiveness while the refrigerator-stable Zostavax can be kept in a refrigerator until it needs to be used.
Also Check: Can You Power Wash Shingles
How Does The Shingles Vaccine Work
The vaccine recommended for most people is a live vaccine called Zostavax. It contains a weakened chickenpox virus . It’s similar, but not identical, to the chickenpox vaccine.
People with a weakened immune system cannot have live vaccines. They will be offered a non-live vaccine called Shingrix. It activates the immune system but also contains an ingredient called an adjuvant, which helps to boost the response to the vaccine.
Very occasionally, people develop chickenpox following shingles vaccination . Talk to a GP if this happens to you.
Conditions Treated By Shingrix And Zostavax
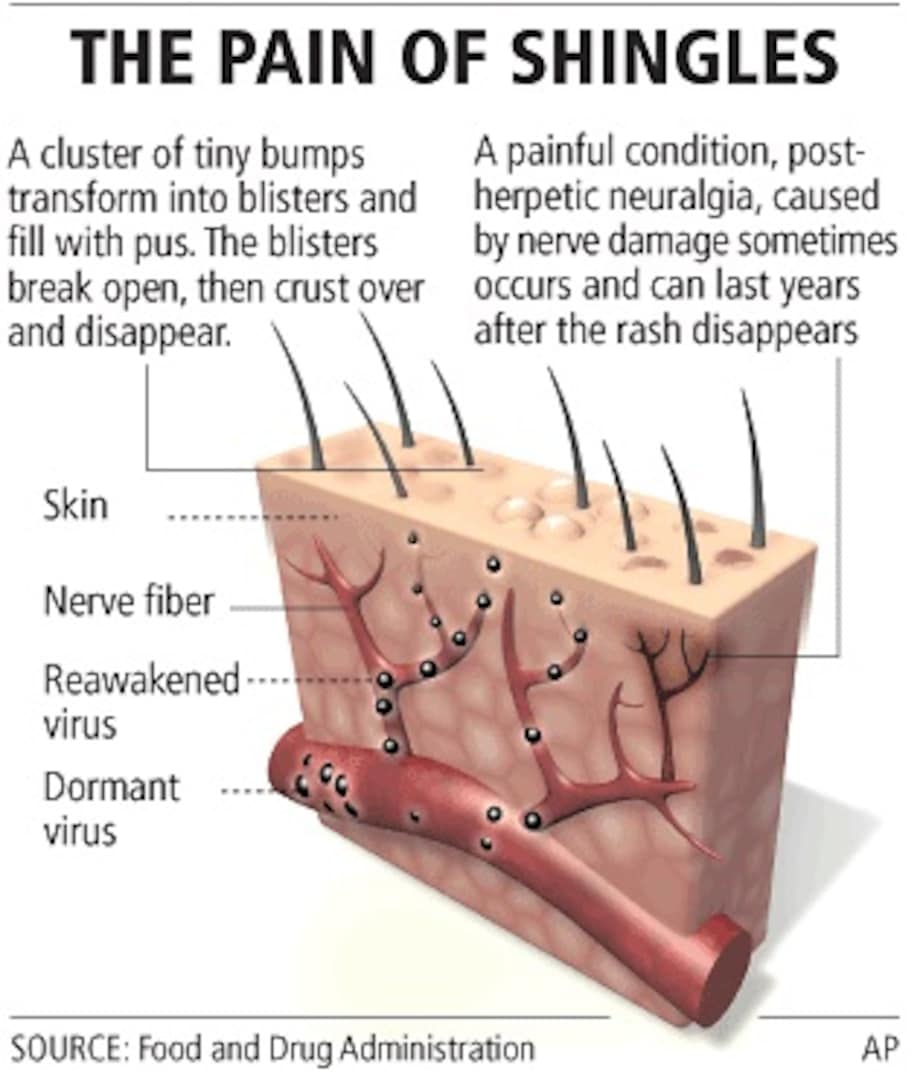
Shingrix and Zostavax are FDA approved to prevent shingles . Both vaccines are indicated to prevent shingles in adults aged 50 years and older. Shingrix and Zostavax are not used to prevent primary varicella infection, also known as chickenpox.
Postherpetic neuralgia is a common type of nerve pain that arises with shingles. Because Shingrix and Zostavax can prevent shingles, they can also prevent postherpetic neuralgia and other painful complications from shingles. However, these vaccines are not labeled to treat PHN.
| Condition |
| Yes |
Recommended Reading: What Stops Shingles From Itching
Who Should Get Zostavax
People 60 years of age or older should get shingles vaccine . They should get the vaccine whether or not they recall having had chickenpox, which is caused by the same virus as shingles. Studies show that more than 99% of Americans aged 40 and older have had chickenpox, even if they dont remember getting the disease. There is no maximum age for getting shingles vaccine.
Two vaccines are licensed and recommended to prevent shingles in the U.S.. Zoster vaccine live has been in use since 2006. Recombinant zoster vaccine , has been in use since 2017 and is recommended by ACIP as the preferred shingles vaccine.
Even if you have had shingles, you can still receive shingles vaccine to help prevent future occurrences of the disease. There is no specific length of time you must wait after having shingles before receiving shingles vaccine, but generally you should make sure the shingles rash has disappeared before getting vaccinated. The decision on when to get vaccinated should be made with your healthcare provider.
Talk with your healthcare provider if you have questions about shingles vaccine. Shingles vaccine is available in doctors offices and pharmacies. To find doctors offices or pharmacies near you that offer the vaccine, visit Zostavax or HealthMap Vaccine Finder.
Development Of A Vaccine Against Zoster Efficacy And Safety
The varicella vaccine provided an important opportunity to explore whether boosting VZV-specific T cell immunity in older adults reduced the risk of VZV reactivation. Early research using the varicella vaccine found that it successfully increased levels of VZV T cell immunity among healthy, older adults , and decreased the incidence and severity of zoster in bone marrow transplant recipients .
Read Also: Is There Always A Rash With Shingles
Very Common And Common Adverse Events
Very common adverse events occur in 10% or more of vaccinees. Common adverse events occur in 1% to less than 10% of vaccinees.
Injection site reactions are very commonly reported for both LZV and RZV. For LZV recipients the frequency is slightly higher in adults aged < 60 years. For all ages, the majority of these events were rated mild or moderate in intensity and lasted less than 2 days.
Due to the adjuvant in RZV, which induces a high cellular immune response and helps address the natural age-related decline in immunity, RZV is more reactogenic than LZV.
Injection site AEs are very commonly reported by recipients of RZV. Approximately 80% report injection-site pain and approximately 30% report redness at the site of injection.
Systemic adverse events, primarily fatigue and myalgia are common in LZV recipients and very common in RZV recipients . For RZV, they include headache .
Local and systemic reactions that were severe enough to interfere with normal activities have been more frequently reported following the receipt of RZV than LZV. However, these reactions have been temporary . Patient education on the short-term reactogenicity of the RZV is recommended prior to vaccine administration to promote adherence to the second dose.
The Problem With Medical Records Tracking Vaccine Schedules
Another obstacle is the fact that it can be difficult for family physicians to know exactly what vaccinations a patient has received. Unlike pediatric patients, who typically have accessible records of their vaccination schedule, it can be trickier for adults.
For adults, it becomes quite challenging, especially when they switch providers, because often times you have to track down records to find out if theyve been vaccinated, said Jain.
She said it can be complicated to try and decipher which vaccines patients have received and which ones they should get without clear records.
For adults over the age of 65, you want to find out if theyve gotten the two pneumonia vaccinations that are recommended, so it becomes a challenge to find out if theyve gotten both, or just one, she said.
Same thing goes now for the shingles vaccination. Youre kind of tracking down records, and when you dont have them, you have to make a clinical judgment.
Despite these challenges, Jain says the fact that Shingrix is a newly minted vaccination may make things a bit more straightforward.
The nice thing about Shingrix is that its so new, most patients have not gotten it, she said. Even if theyve had Zostavax, its recommended that they get Shingrix in addition to it, so thats a little bit less of a challenge with a new vaccination.
Also Check: How To Figure How Many Shingles You Need
Persons With Chronic Diseases
Autoimmune disease
Although definitive data are lacking, individuals with autoimmune disease not being treated with immunosuppressive drugs are not considered significantly immunocompromised. Individuals 50 years of age without contraindications should receive RZV.
For more information, refer to Immunization of Immunocompromised Persons, and Immunization of Persons with Chronic Diseases in Part 3.
Coadministration With Influenza Vaccine
A total of 828 patients were included in the study, 413 in the co-administration group and 415 in the control group. Among coadministration patients, VRR was 95.8% , and anti-gE GMC ratio was 1.08 . Immunogenicity to the IIV4 vaccine was also noninferior between the groups. This study demonstrated that immune responses to both HZ/su and IIV4 were not affected by coadministration.12
Recommended Reading: Can You Get Shingles Without Having Chicken Pox
Unanswered Questions About Varicella Vaccine
3.3.1. Optimal dose schedule
When varicella vaccine is included as part of the universal childhood immunization schedule, it is unclear whether one or two doses is preferable. A one-dose schedule has been shown to be highly effective in reducing severe disease, but breakthrough cases of mild varicella occur. Studies comparing immune responses following a one- or two-dose regime have shown that higher seroconversion rates and a higher antibody titer are achieved among subjects who receive two vaccine doses, regardless of the time interval between doses . Nevertheless, cost-effectiveness modeling of two- versus one-dose schedules, suggesting that addition of a second dose demonstrates unfavorable incremental cost-effectiveness, has been instrumental in informing Australias decision to implement a one-dose schedule . More recent modeling suggests that improving vaccine coverage, for example, from 83% to 95% by age 24 months, results in the incremental benefit of a second dose falling by 70% . As well as being cheaper to implement, potential advantages to a one-dose schedule include the persistence of low levels of circulating wild-type varicella, which would prevent a shift of varicella into older age groups who are at greater risk of complications. Persisting varicella circulation would also provide exogenous boosting to maintain levels of immunity in older people, and theoretically prevent a rise in the incidence of zoster cases .
Annual Updates To The Immunization Schedule 1995 To 2010
As more vaccines became available, an annual update to the schedule was important because of changes that providers needed to know, such as detailed information about who should receive each vaccine, age of receipt, number of doses, time between doses, or use of combination vaccines. New vaccines were also added.
Important changes to the schedule between 1995 and 2010 included:
- New vaccines: Varicella , rotavirus hepatitis A pneumococcal vaccine
- Additional recommendations for existing vaccines: influenza hepatitis A
- New versions of existing vaccines: acellular pertussis vaccine intranasal influenza
- Discontinuation of vaccine: Oral polio vaccine
2000 | Recommended Vaccines
* Given in combination as DTaP** Given in combination as MMR
Don’t Miss: What Foods Should You Avoid With Shingles
Coverage And Cost Comparison Of Shingrix Vs Zostavax
For adults aged 50 years and older, only plans with Medicare Part D coverage will cover the Shingrix vaccine. However, there may still be a copay even with Medicare Part D coverage. The average cash price for one Shingrix dose is $167, though you may be able to use a prescription discount card to lower this cost. Check with your local pharmacy to see if you can use a Shingrix SingleCare card.
Like Shingrix, Zostavax is primarily covered by Medicare Part D plans or Medicare Advantage plans with Medicare Part D coverage. The copay for Zostavax with insurance can vary. With an average cash price of $278, Zostavax can be expensive with or without insurance. Using a prescription discount card for Zostavax may be able to reduce this cost.
| * |
*not reportedFrequency is not based on data from a head-to-head trial. This may not be a complete list of adverse effects that can occur. Please refer to your doctor or healthcare provider to learn more.Source: DailyMed , DailyMed
Herpes Zoster Subunit Vaccine
Recent development of a new recombinant subunit vaccine, HZ/su , with impressive efficacy has the potential to transform current zoster vaccination policy. Application for its approval was submitted to the FDA, the European Medicines Agency and Health Canada at the end of 2016. HZ/su consists of 50 µg of recombinant VZV antigen which directs the immune response to the virus itself, combined with the AS01B adjuvant system to stimulate T cell immunity to recombinant proteins . As described in detail elsewhere , two very large multicenter-blinded RCTs reported that, among participants who had received two doses of Hz/su, VE against zoster was 97.2% for adults aged 50 years and 89.8% for those aged 70 years . In a pre-specified analysis of data pooled from both trials, the VE against PHN was 88.8% .
While systemic reactions were more commonly reported for the HZ/su vaccine than Zostavax , these adverse effects were transient and around 95% of participants receiving HZ/su in both trials received both vaccine doses. Reassuringly, serious adverse events, immune-mediated diseases and deaths across the entire study period were reported equally between the vaccinated and placebo groups in both ZOE trials. Initial results from a multi-country randomized trial among 828 adults aged 50 years, suggest that coadministration of HZ/su with an influenza vaccine is well tolerated .
You May Like: Where Can Shingles Appear On The Body
How Well Does Zostavax Work
Zostavax®, the shingles vaccine, reduced the risk of shingles by 51% and the risk of post-herpetic neuralgia by 67% based on a large study of more than 38,000 adults aged 60 years or older. Protection from shingles vaccine lasts about 5 years.
While the vaccine was most effective in people 60 through 69 years old, it also provides some protection for people 70 years old and older.
Adults vaccinated before age 60 years might not be protected later in life when the risk for shingles and its complications are greatest.
Cautionary Statement Regarding Forward
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described in the Company’s Annual Report on Form 20-F for 2020 and any impacts of the COVID-19 pandemic.
Also Check: Can You Get Shingles From The Vaccine
Side Effects And Counseling For Reactogenicity
In eight clinical trials of more than 10,000 immunocompetent participants 50 years or older, grade 3 reactions were common after patients received Shingrix. About 1 out of 10 adults who received Shingrix reported grade 3 injection-site symptoms such as pain, redness, and swelling. Also, about 1 out of 10 reported grade 3 systemic reactions such as myalgia, fatigue, headache, shivering, fever, and gastrointestinal illness. Most people who got Shingrix reported at least some pain at the injection site.
Local and systemic grade 3 reactions among immunocompromised adults were evaluated in six studies in five immunocompromised groups. Local grade 3 reactions occurred in 10.7% to 14.2% of RZV recipients, and systemic grade 3 reactions occurred in 9.9% to 22.3% of RZV recipients, compared with 0% to 0.3% and 6.0% to 15.5%, respectively, among placebo recipients. The most commonly reported systemic symptoms were fatigue and myalgia.
Healthcare providers should counsel patients about expected reactogenicity before administering Shingrix.
What to tell patients about the side effects of Shingrix:
Most people have a sore arm after they get Shingrix. Many people have redness and swelling on their arm spanning several inches where they got the shot. Many people also feel tired or have muscle pain, a headache, shivering, fever, stomach pain, or nausea.
Cdc Recommends New Shingles Vaccine To Replace Older One
Shingrix is recommended over Zostavax, the existing shingles vaccine
Revaccination with Shingrix is recommended for people who have received Zostavax
A new adult vaccine has received a double thumbs-up from the American federal health system.
On the heels of the Food and Drug Administration approval of Shingrix, a new vaccine from GlaxoSmithKline for the prevention of shingles, a federal committee of immunization experts voted Wednesday to recommend Shingrix for all Americans 50 and older.
Shingles, also known as herpes zoster, is a painful, itchy rash that develops on one side of the body and can last for two to four weeks. One in three Americans will develop shingles in their lifetime, with the risk increasing to half of adults over 85, according to the US Centers for Disease Control and Prevention.
The Advisory Committee on Immunization Practices, which advises the CDC on vaccine usage, also recommended that adults who received Zostavax, a shingles vaccine made by Merck, be revaccinated with Shingrix.
Additionally, the committee expressed its preference for Shingrix over Zostavax.
GlaxoSmithKline says Shingrix will be available shortly.
Zostavax was licensed and recommended by the committee in 2006 for people 60 and older, including those who have had an episode of shingles. Until now, it has been the only approved vaccine to protect against the virus.
Also Check: What Does A Light Case Of Shingles Look Like