Why Is The Shingles Vaccine Important
Shingles causes a painful rash and blisters and it can lead to serious complications. The most common complication is post-herpetic neuralgia , a condition that causes burning pain that can last long after the shingles rash and blisters go away. The older you are when you get shingles, the more likely you are to develop PHN.
Getting vaccinated is the best way to prevent shingles and PHN.
Shingles is caused by the same virus that causes chickenpox. After you have chickenpox, the chickenpox virus stays dormant in your body. The virus can activate years later and cause shingles.
Symptoms of shingles include:
Shingles cant spread from person to person like chickenpox. But if you have shingles, you can spread the virus to someone who isnt immune to chickenpox meaning someone who hasnt had chickenpox and isnt vaccinated against it. If that happened, the person might get chickenpox but not shingles. Learn more about shingles.
- Adults age 50 and older
- Adults 19 years and older who have a weakened immune system because of disease or treatments
You need to get 2 doses of Shingrix. Youll need the second dose 2 to 6 months after the first dose. You need to get Shingrix even if you:
- Have already had shingles
- Have been vaccinated against shingles with Zostavax
- Are not sure if youve had chickenpox
When Should I See A Doctor Because Of The Side Effects I Experience From Shingrix
Shingrix causes a strong response in your immune system, so it may produce short-term side effects. These side effects can be uncomfortable, but they are expected and usually go away on their own in 2 or 3 days. You may choose to take over-the-counter pain medicine such as ibuprofen or acetaminophen. Contact your healthcare provider if the symptoms are not improving or if they are getting worse.
In clinical trials, Shingrix was not associated with serious adverse events. In fact, serious side effects from vaccines are extremely rare. For example, for every 1 million doses of a vaccine given, only one or two people might have a severe allergic reaction. Signs of an allergic reaction happen within minutes or hours after vaccination and include hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, or weakness. If you experience these or any other life-threatening symptoms, see a doctor right away.
Side Effects Of The Shingles Vaccine: Is It Safe
Shingles is a painful rash caused by varicella zoster, the same virus responsible for chickenpox.
If you had chickenpox as a child, the virus hasnt completely gone away. It hides dormant in your body and can reemerge many years later as shingles.
About 1 in 3 people in the United States will develop shingles in their lifetime. This is why vaccination is important. But you should also be prepared for possible side effects. In this article, well discuss the side effects, and talk about who should get the vaccine.
Older adults are most likely to develop shingles. This is why the shingles vaccine is recommended for people ages 50 and older.
Shingrix is the only shingles vaccine approved by the U.S. Food and Drug Administration .
The Shingrix vaccine is a recombinant vaccine. This means vaccine manufacturers created it by altering and purifying DNA that creates an immune response to fight the virus.
The CDC recommends Shingrix for the prevention of shingles and related complications. The Shingrix vaccine is also recommended for anyone who has already gotten another type of shingles vaccine.
Currently, the CDC recommends healthy people ages 50 and older get the Shingrix vaccine. Doctors administer the vaccine in two doses, which are given 2 to 6 months apart.
The Shingrix vaccine has high success rates in protecting people against shingles.
The Shingrix vaccine is as much as effective in preventing shingles. The same is true for Shingrix and postherpetic neuralgia.
Recommended Reading: What Helps Nerve Pain From Shingles
Influenza Vaccination Of Persons With A History Of Egg Allergy
Severe allergic and anaphylactic reactions can occur in response to a number of influenza vaccine components, but such reactions are rare . All but the recombinant inactivated influenza vaccine may have come into contact with egg protein. The use of influenza vaccines for persons with a history of egg allergy has been reviewed recently by ACIP . VAERS data mining did not identify a higher than expected proportion of serious allergic events after influenza vaccination during the 2011-2012 season, relative to all other reported vaccines and adverse events in the database. Persons with a history of egg allergy should receive recombinant inactivated vaccine , or IIV.
Other measures, such as dividing and administering the vaccine by a 2-step approach and skin testing with vaccine, are not recommended .
All vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available. ACIP recommends that all vaccination providers be certified in cardiopulmonary resuscitation , have an office emergency plan, and ensure that all staff are familiar with the plan . Some persons who report allergy to egg might not be egg-allergic. Those who are able to eat lightly cooked egg without reaction are unlikely to be allergic.
A previous severe allergic reaction to influenza vaccine, regardless of the component suspected to be responsible for the reaction, is a contraindication to future receipt of the vaccine .
Why Do Vaccines Cause Fevers

Vaccines prepare the immune system to protect against viruses or bacteria that could make people sick. The way this happens is that they introduce components of the germs that are known to activate the immune response. However, vaccines will not cause a significant enough immune response that the person suffers untoward events, such as can occur during natural infections. With this said, in some cases the immune response is strong enough to cause detectable symptoms, like a mild fever.
Knowing that vaccines can cause a fever, sometimes parents wonder if a lack of fever means the vaccine is not working. However, not everyone who responds to a vaccine will develop a fever.
Also Check: List Of Class 4 Impact Resistant Shingles
Side Effects Of Shingles Vaccine
The most common shingles shot side effects include pain and soreness at the injection site. Some people also notice a bit of redness, swelling or itching at the site of the shot. Other side effects of the shingles vaccine may include fatigue, muscle pain, headache, shivering, fever, stomach pain, or nausea. According to the CDC, side effects are more common in younger people than older people.
Most people can resume their regular activities immediately after vaccination. However, about 1 out of 6 people develop flu-like symptoms that last anywhere from 1 to 3 days.
Side effects can occur after the first, second, or both doses of Shingrix vaccine. If possible, it is a good idea to schedule vaccination the day before some downtime, so you can rest if you develop side effects.
If you develop flu-like symptoms after shingles vaccination, you can take ibuprofen or acetaminophen to control your fever and improve comfort. Those who develop flu-like symptoms after their first dose of vaccine may want to pre-medicate with ibuprofen or acetaminophen an hour or so before their second dose. Your healthcare provider can answer your questions about shingles vaccine side effects and pre-medication for vaccination.
Serious side effects are rare, but not impossible, after shingles vaccination. If you develop hives, swelling of the face or throat, difficulty breathing, a rapid heartbeat, or sudden dizziness or weakness, and seek medical care immediately.
Serious Side Effects Of Shingrix
Along with its needed effects, zoster vaccine, inactivated may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor immediately if any of the following side effects occur while taking zoster vaccine, inactivated:
Also Check: What Is Shingles Is It Contagious
Is The Shingles Vaccine Safe
According to the CDC, research has shown that Shingrix is safe.
Some people experience short-term adverse effects, such as a fever, muscle aches, and headaches. However, these usually last only 2â3 days .
In rare cases, people have developed Guillain-Barré syndrome after having the shingles vaccine. However, this can also happen after shingles. GBS is a severe nervous system disorder.
The recommends Shingrix for people who have or are likely to have a weakened immune system due to a health condition or treatment. Having a weakened immune system increases a personâs risk of developing shingles.
It can happen with the following:
- a medical condition that compromises the immune system, such as AIDS
- cancer that affects the lymphatic system or bone marrow
- cancer treatments, such as radiation or chemotherapy
- medications that affect the immune system, such as steroids
However, a person with a weakened immune system should speak with their doctor about whether to have the vaccine and when.
Side Effects And Counseling For Reactogenicity
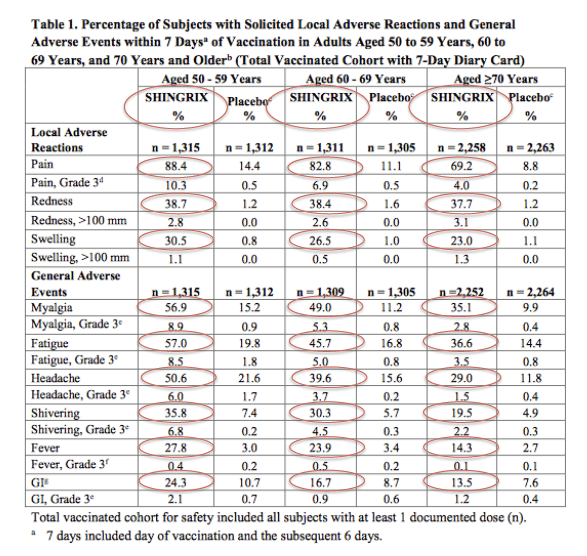
In eight clinical trials of more than 10,000 immunocompetent participants 50 years or older, grade 3 reactions were common after patients received Shingrix. About 1 out of 10 adults who received Shingrix reported grade 3 injection-site symptoms such as pain, redness, and swelling. Also, about 1 out of 10 reported grade 3 systemic reactions such as myalgia, fatigue, headache, shivering, fever, and gastrointestinal illness. Most people who got Shingrix reported at least some pain at the injection site.
Local and systemic grade 3 reactions among immunocompromised adults were evaluated in six studies in five immunocompromised groups. Local grade 3 reactions occurred in 10.7% to 14.2% of RZV recipients, and systemic grade 3 reactions occurred in 9.9% to 22.3% of RZV recipients, compared with 0% to 0.3% and 6.0% to 15.5%, respectively, among placebo recipients. The most commonly reported systemic symptoms were fatigue and myalgia.
Healthcare providers should counsel patients about expected reactogenicity before administering Shingrix.
What to tell patients about the side effects of Shingrix:
Most people have a sore arm after they get Shingrix. Many people have redness and swelling on their arm spanning several inches where they got the shot. Many people also feel tired or have muscle pain, a headache, shivering, fever, stomach pain, or nausea.
You May Like: What Does Shingles Look Like And Feel Like
Another Jab At Shingles
In October 2018, the FDA approved Shingrix, a two-shot shingles vaccine for patients 50 and older. To be the most effective, patients must get the second shot between two to six months after the first. Clinical trials demonstrated that it was 91% to 97% effective in preventing shingles, and that protection seems to stay strong, at least for the first four years in the patients who were tracked.
There has slowly been uptick, but still very large group of those aged 50 and older who have not received it, saysNatalie Baker, DNP, president of the gerontological advanced practice nursing association .
Given its improved efficacy and the fact that the efficacy of Zostavaxwanes over the course of a few years, regulators recommended getting the Shingrix shots even if you already received Zostavax, which was discontinued in 2020. A lot of our older adults have received Zostavax, but, unfortunately, it just does not continue to be effective, she says, explaining that even those patients should get the Shingrix vaccine.
How Cdc Monitors Vaccine Safety
CDC and FDA monitor the safety of vaccines after they are approved or authorized. If a problem is found with a vaccine, CDC and FDA will inform health officials, health care providers, and the public.
CDC uses 3 systems to monitor vaccine safety:
- The Vaccine Adverse Event Reporting System : an early warning system, co-managed by CDC and FDA, to monitor for potential vaccine safety problems. Anyone can report possible vaccine side effects to VAERS.
- The Vaccine Safety Datalink : a collaboration between CDC and 9 health care organizations that conducts vaccine safety monitoring and research.
- The Clinical Immunization Safety Assessment Project: a partnership between CDC and several medical research centers that provides expert consultation and conducts clinical research on vaccine-associated health risks.
Don’t Miss: Shingles On Eye And Face
Patient Reports Of Side Effects
During the first eight months of Shingrixs post-marketing use, the Vaccine Adverse Event Reporting System, or VAERS, received 4,381 total reports of adverse events of these 130 were serious.
For every 100,000 doses distributed, the CDC found 136 complaints filed in the system. Approximately 3.2 million doses were distributed by GlaxoSmithKline during the eight-month period of reporting analyzed by the CDC.
Fever, chills and body aches and pain, swelling and redness in the arm receiving the shot were common side effects.
Yet seven patients died within six hours to six weeks of receiving Shingrix, the CDC said. The cause of four of these deaths was cardiovascular disease . Two were immunosuppressed patients who died of sepsis. And one 86-year-old woman died after a fall. v An eighth death after the use of Shingrix was also reported to VAERS, though this was not confirmed by the CDC.
Dr. Elisabeth M. Hesse, lead author of the report and a medical officer in the CDCs Office of Immunization Safety, wrote in an email that no information in medical documentation indicated the reported deaths were related to vaccination.
She noted that the Rotashield vaccine for infants was withdrawn from the market after reports to VAERS of bowel obstruction and an investigation that verified these claims.
Recommended Reading: What Medication Is Prescribed For Shingles
Talk With Your Health Care Provider
Tell your vaccination provider if the person getting the vaccine:
- Has had an allergic reaction after a previous dose of recombinant shingles vaccine, or has any severe, life-threatening allergies
- Is currently experiencing an episode of shingles
In some cases, your health care provider may decide to postpone shingles vaccination until a future visit.
People with minor illnesses, such as a cold, may be vaccinated. People who are moderately or severely ill should usually wait until they recover before getting recombinant shingles vaccine.
Your health care provider can give you more information.
Don’t Miss: Where To Get The Shingles Shot
What Vaccines Can Help Prevent Shingles
There is currently one vaccine available in the U.S. to prevent shingles. Shingrix was approved in 2017 and it is more than 90% effective in preventing shingles. With Shingrix, you get two shots between 2 and 6 months apart and protection lasts an estimated 4-5 years. Doctors recommend it for healthy people over 50 as well as those 19 years of age and older who are or will be immunodeficient or immunosuppressed due to disease or therapy..
An earlier vaccine called Zostavax was removed from the market in 2020. That vaccine used a weak form of the chickenpox virus to send your bodyâs immune system into action to fight the disease. Shingrix does not. If you received the Zostavax vaccine, it is recommended that you also receive Shingrix.
Side Effects Of Getting The Vaccine
Like all vaccines, the shingles vaccines can cause side effects, but they’re generally mild and do not last long.
Common side effects that occur in at least one in 10 people are:
- redness, pain, swelling, itching at the injection site
If the side effects continue for more than a few days, contact your GP or practice nurse.
Tell your GP if you develop a rash after having the shingles vaccination.
Read Also: Is Apple Cider Vinegar Good For Shingles
Learn More Aboutzostavax Lawsuits
Side effects of the shingles vaccine Zostavax may result in the development of a painful and persistent strain of shingles
Shingrix was introduced in October 2017, and has been approved for prevention of shingles among adults age 50 and older. It was the second shingles vaccine approved in the U.S., and has been widely viewed as a superior replacement for Mercks Zostavax , which has been linked to complications as a result of an under-attenuated live virus contained in the vaccine.
The MMWR findings come from data lifted from the Vaccine Adverse Event Reporting System during the first eight months Shingrix was in use, from October 20, 2017 to June 30, 2018, resulting in the distribution of 3.2 million doses. The most common adverse events were fever, injection site pain, and injection site erythema. The report does not highlight any specific side effects which stood out among the reports of serious adverse events. However, the report does indicates that 196 patients developed shingles even after receiving the vaccine, though the CDC indicates that it believes 14 of those patients may have already been suffering from a shingles outbreak when they received the shot.
All but one of those patients suffered injection site reactions, including pain, erythema, and pruritus.
As of this latest MMWR report, the number of reported injection errors had risen to 230.
What Is The Process
The special master’s decision may be appealed and petitioners who reject the decision of the court may file a claim in civil court against the vaccine company and/or the health care provider who administered the vaccine.
Recommended Reading: Owens Corning Shingles For Sale